How to Determine Which Solvent to Use in Recrystallization
A watch glass with some ice cubes on top of the Erlenmeyer flask allows you to gently reflux the mixture less solvent evaporates away during the heating phase. The first step of recrystallization is a solvent selection procedure based on properties like.
Briefly explain how to do TLC.

. The criteria used to choose an appropriate recrystallization solvent includes. What is meant by reflux and what purpose does it serve. How do you determine how much solvent to use in a recrystallization.
What are the properties of a perfect recrystallization solvent. Place about 50 mg of the sample in a test tube. Moreover it is desirable that the boiling points of the two solvents should be.
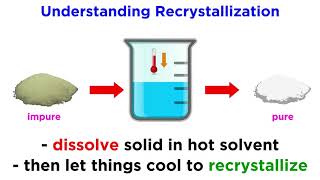
Depending on its molecular structure a solute can be classified as soluble partially soluble or insoluble in these solvents or solvent mixture. A finding a solvent with a high temperature coefficient. A good recrystallization solvent should 1 dissolve a moderate quantity of the substance being purified at an elevated temperature but only a small quantity at low temperatures 2 not react with the substance being purified 3 dissolve impurities readily at a low temperature or not dissolve them at all and 4.
Place about 50 mg of the sample in a test tube. This should be done in a suitable solvent as determined in part 1 and at high temperature boiling point of the solvent. Following the experiment you will calculate the percent recovery and determine the melting point of each product.
G solid recovered g. This testing can be accomplished by putting a small amount of your solute about the size of a pea into three small test tubes. Add a small amount of solvent to the test tube.
Similarly you may ask what is solvent pair recrystallization. Place a few crystals of your unknown in a small test tube. How do you determine how much solvent to use in a recrystallization.
The following is an outline of the recrystallization process. Purchase and Disposal Cost. A finding a solvent with a high temperature coefficient.
How do you determine the amount of recrystallization solvent to use. The second solvent solvent 2 should induce crystallization when added to a saturated solution of your compound in the primary solvent. You determine what small means c.
Out solubility tests to determine a suitable solvent. The ideal solvent for recrystallization is one in which the solute has high solubility at high temperature and low solubility at low temperature. Procedure for Determining a Recrystallization Solvent Place about 50 mg of the sample in a test tube.
In each test tube place 05 mL of each potential solvent. Briefly explain the difference between precipitation and crystallization 4. If the sample dissolves completely the solubility in the cold solvent is too high to be a good recrystallization solvent.
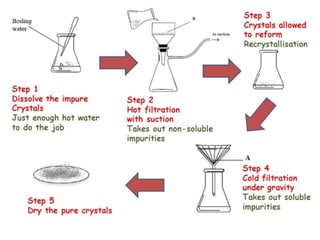
To dry the crystals leave them in the filter funnel and draw air through them for several minutes. If the solvent is not specified you will need to test a variety of solvents to determine what will work best for the solute you are trying to recrystallize. 5 rinse the crystals with a.
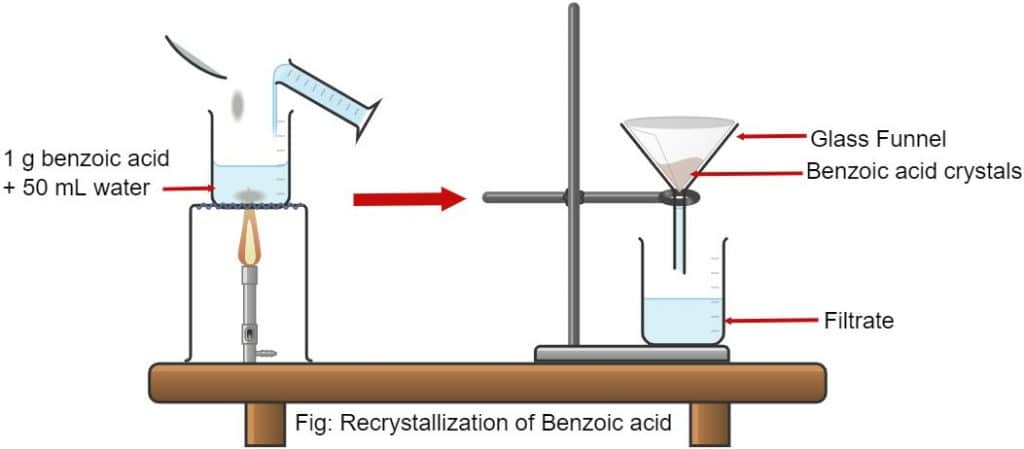
The criteria used to choose an appropriate recrystallization solvent includes. If a recrystallization solution has been allowed to cool and crystals have not formed it may be due to a condition known as supersaturation. Using a hot plate dissolve approximately 10 g of impure benzoic acid in 30 35 mL of hot water water at or near its bp in a 125 mL Erlenmeyer flask.
Occasionally crystals will not form even though the saturation point has been reached. Solvent should be used for the recrystallization and a MINIMUM of ICE-COLD solvent should be used for the rinse. DO NOT SELL MY INFO.
If there is a residual amount of material that does not dissolve upon adding a small amount of additional solvent H2O do not continue to add more. Rinse the crystals on the Büchner funnel with a small amount of fresh cold solvent the same solvent used for recrystallization to remove any impurities that may be sticking to the crystals. In many cases it takes some time to dissolve all the crude product.
2 dissolve the solute in a minimum of near- boiling solvent. For a two-solvent recrystallization you should have one solvent solvent 1 in which your desired compound is soluble at the boiling point. 2 Determine what is a suitable solvent for your unknown.
Percent solid lost in cold water solubility in cold water solubility in hot water x100. Add about 05 mL of cold solvent. If the solid dissolves at room temperature select a new solvent.
Add about 05 mL of cold solvent. If you have to do that you might include mixed solvents. 4 collect the crystals by filtration.
The solvent must not dissolve the compound at low. Show a SN2 mechanism. You could try to look up solubilities in chemistry handbooks or experiment with different solvents.
If the sample dissolves completely the solubility in the cold solvent is too high to be a good recrystallization solvent. In the introductory organic lab course the solvent for recrystallization is usually determined for you. Add about 05 mL of cold solvent.
Lost in cold solvent solubility in cold solventsolubility in hot solvent x100 recovery of solid g solid g solid lost x 100 g solid Example 1 - The solubility of solid X in hot water 550 g100 ml at 100 oC is not very great and its solubility in cold water 053 g100ml at. In the above cases the amounts of both solvents should be kept to the minimum to a avoid the unnecessary use of too much solvent and b recrystallization from dilute solution is very inefficient. The solvent must not dissolve the compound at low temperatures that includes room temperature but must dissolve the.
1 Pick the solvent. 3 allow the solution to cool slowly and undisturbed to room temperature RT then possibly to ice temperature. If the sample does not dissolve in the cold solvent heat the test tube until the solvent.
Macroscale Crystallization In the first part of the procedure one gram of acetanilide will be purified by recrystallization. 053550 x100 964. Grams solid lost in cold water grams mass of original solid x percent lost 500 g x 964 0482 g.
Show a SN1 mechanism. If the sample dissolves completely the solubility in the cold solvent is too high to be a.

A Hated Method For Purifying Compounds Column Chromatography A Lot Solvent A Lot Silica And A Lot Time Is Neede Organic Chemistry Chemistry Labs Chemistry

4 Recrystallization And Melting Points

Pictures From An Organic Chemistry Laboratory Recrystallization Of Benzoquinone From High Boiling Point Petroleum E Organic Chemistry Chemistry Chemistry Labs
Recrystallization Types Procedure Applications Psiberg

Recrystallization Separate Product From Impurities

Recrystallization Separate Product From Impurities
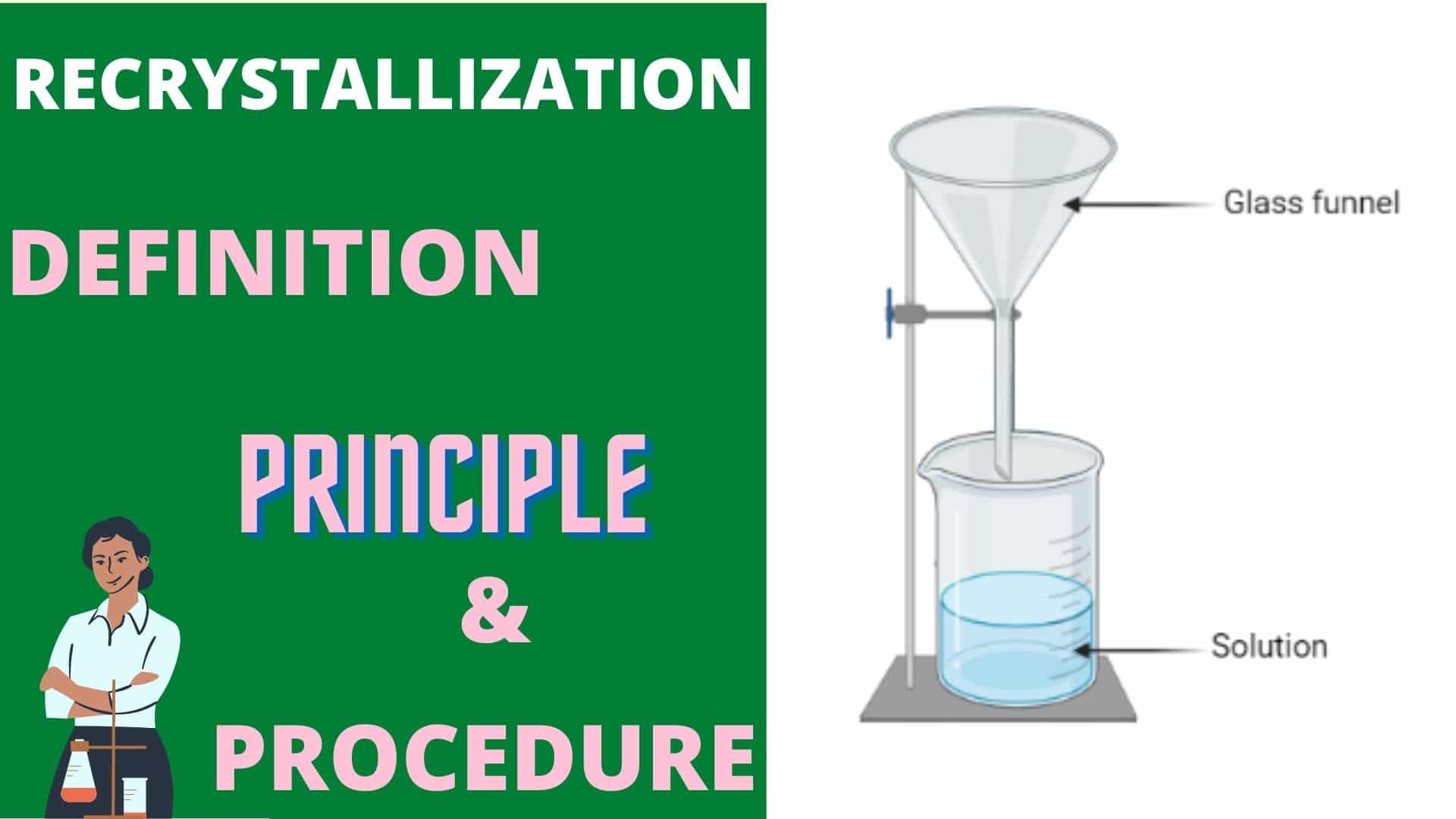
Recrystallization Definition Meaning Chemistry Notes

Recrystallization Separate Product From Impurities

Recrystallization Separate Product From Impurities
Recrystallization Types Procedure Applications Psiberg
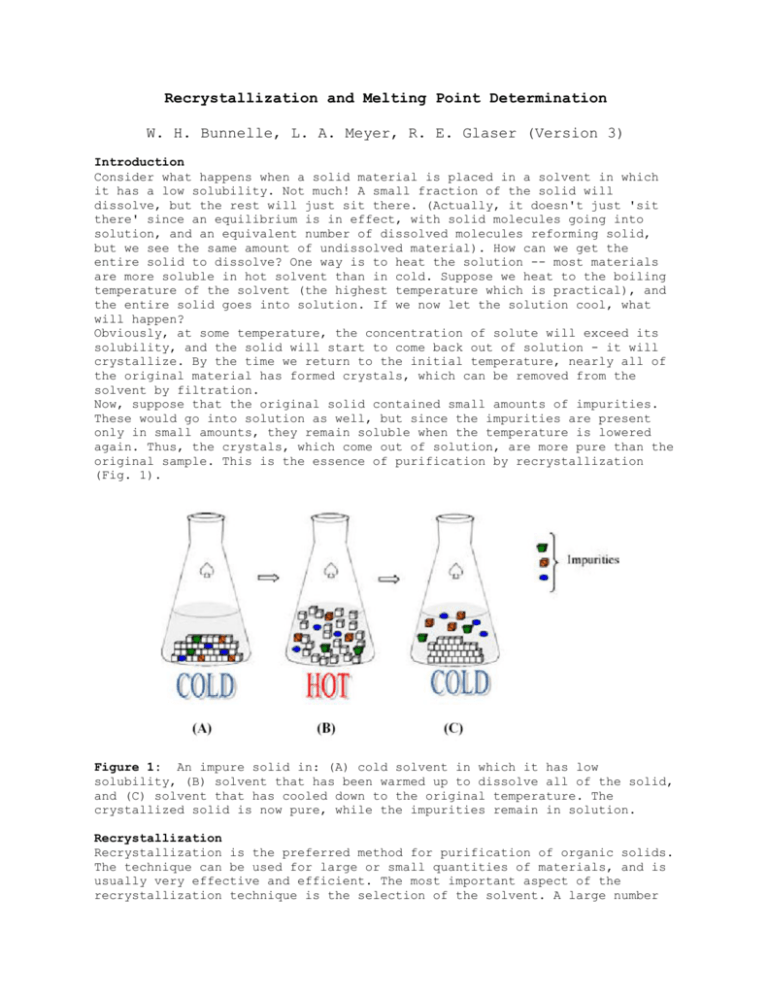
Recrystallization Of Impure Acetanilide And
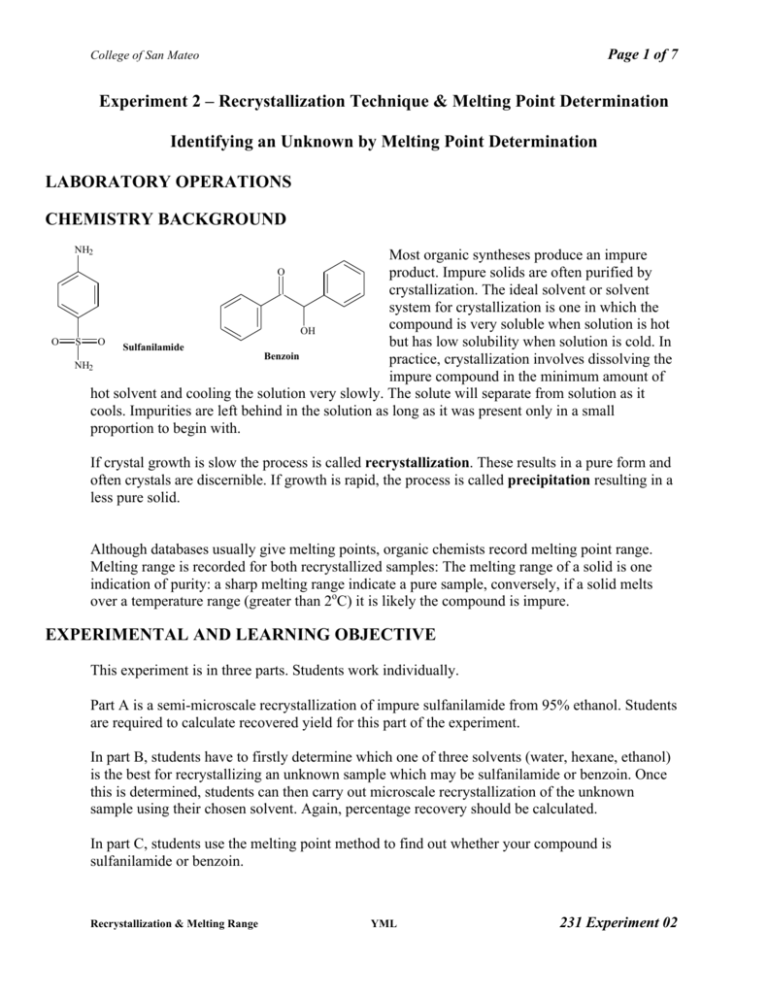
Exp2 Recrystallization And Melting Point Procedure

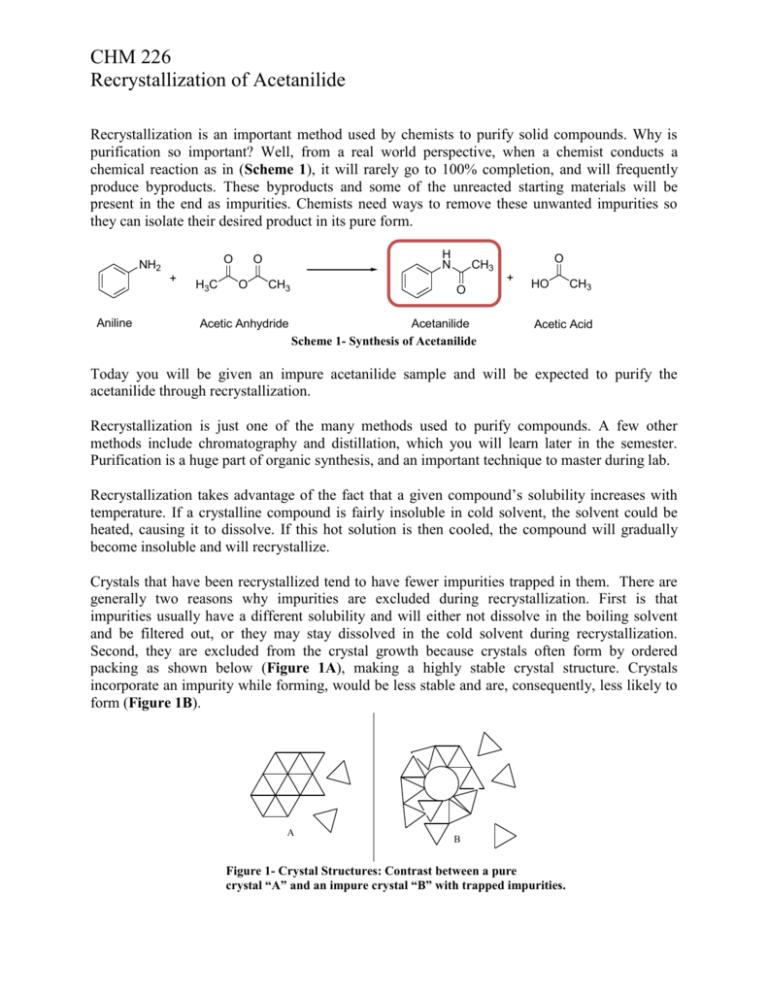


Comments
Post a Comment